——本文经《美国外科病理学杂志》授权发布,其他媒体转载或引用须经《美国外科病理学杂志》同意,否则追究法律责任。
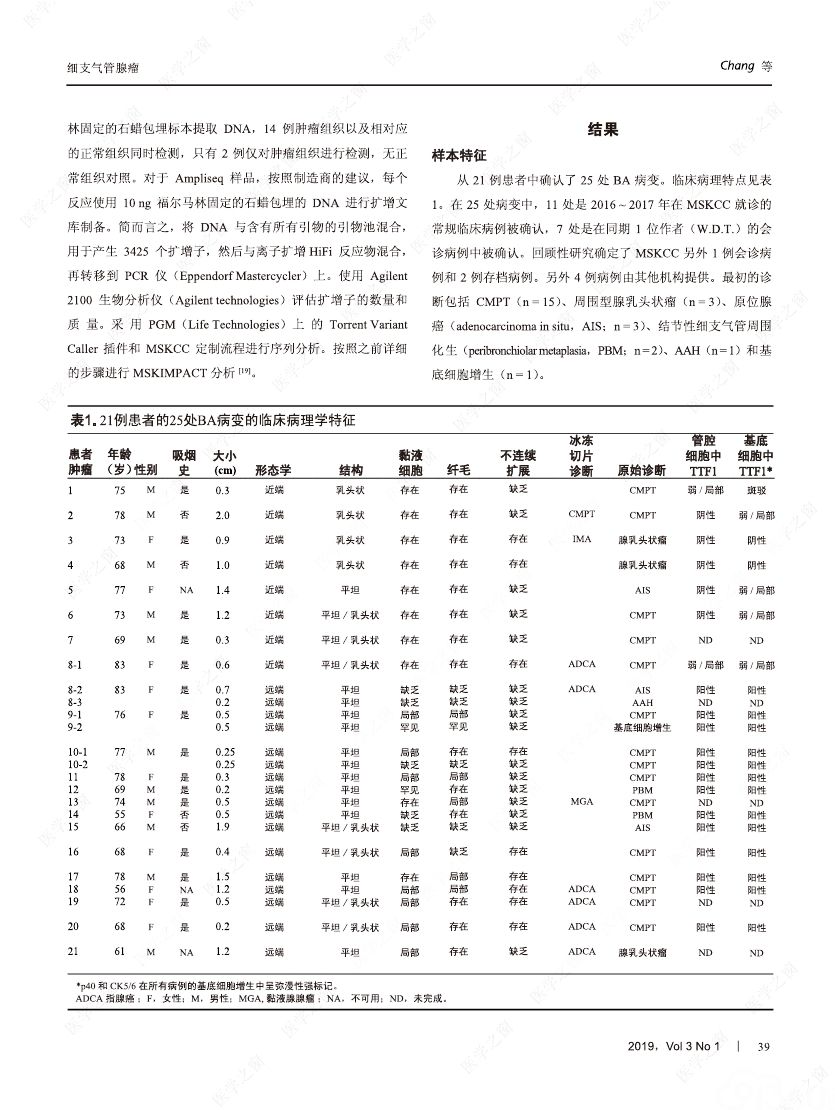
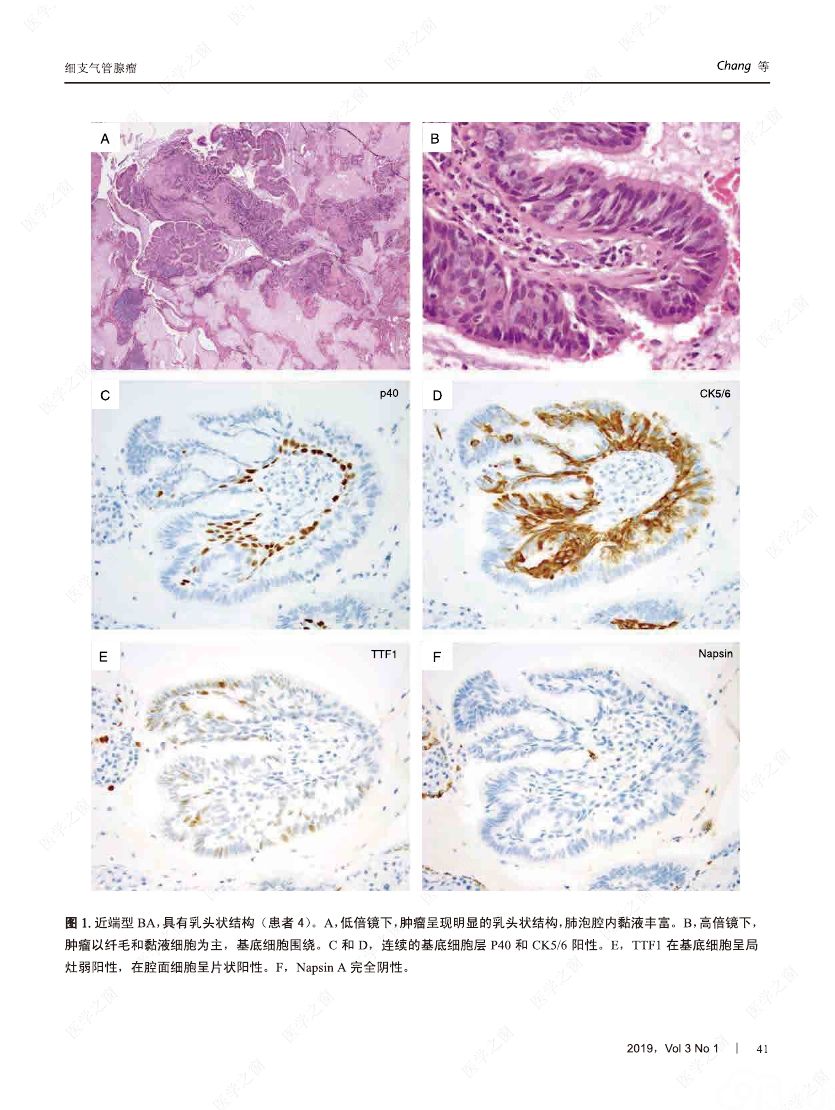
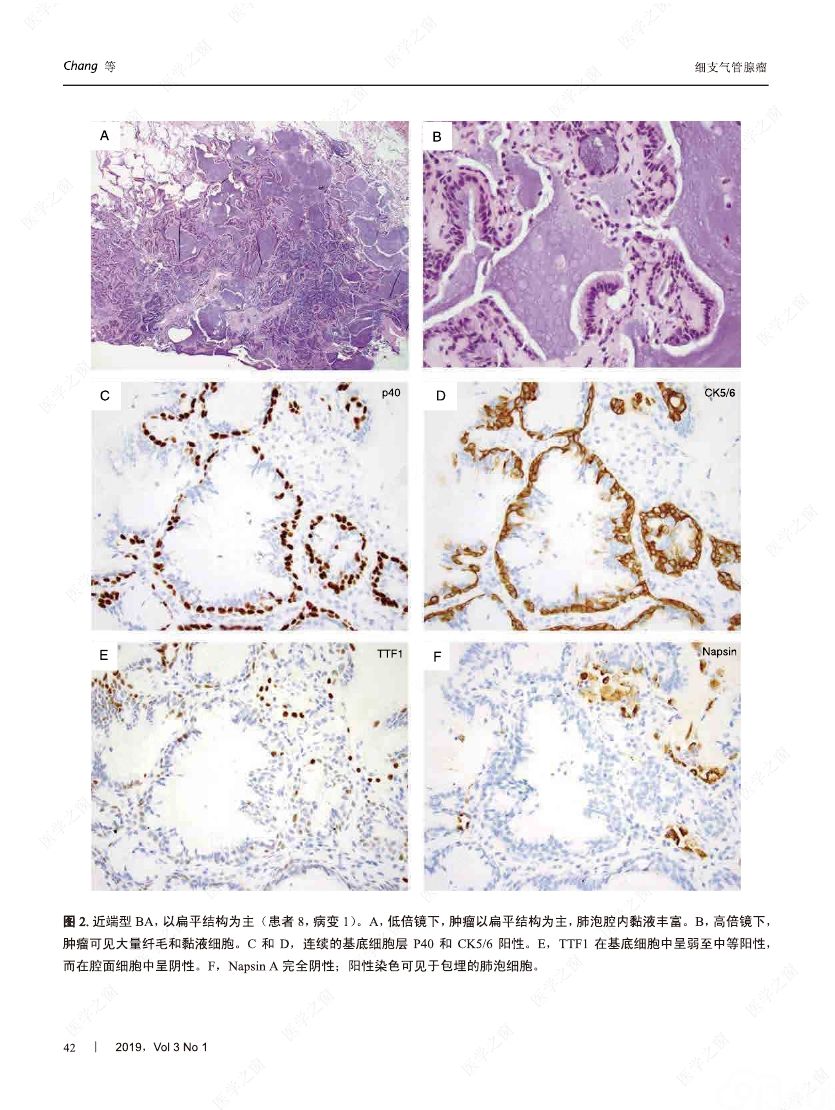
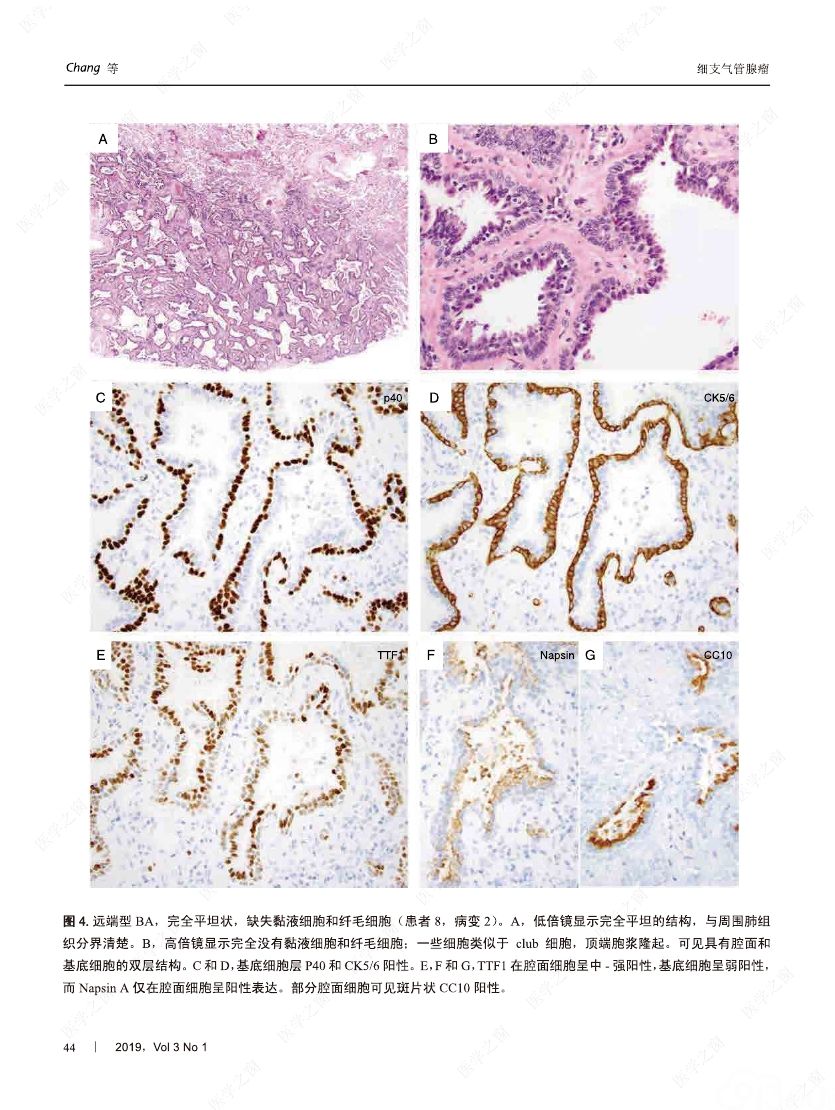
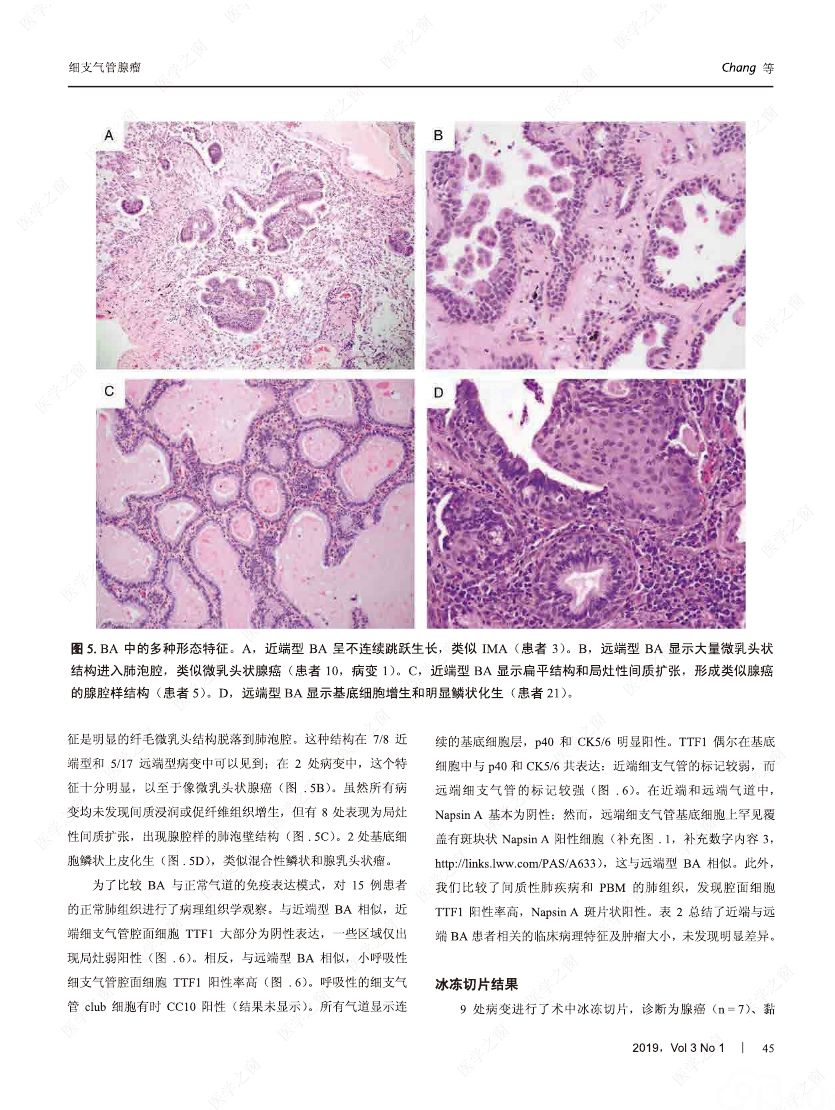
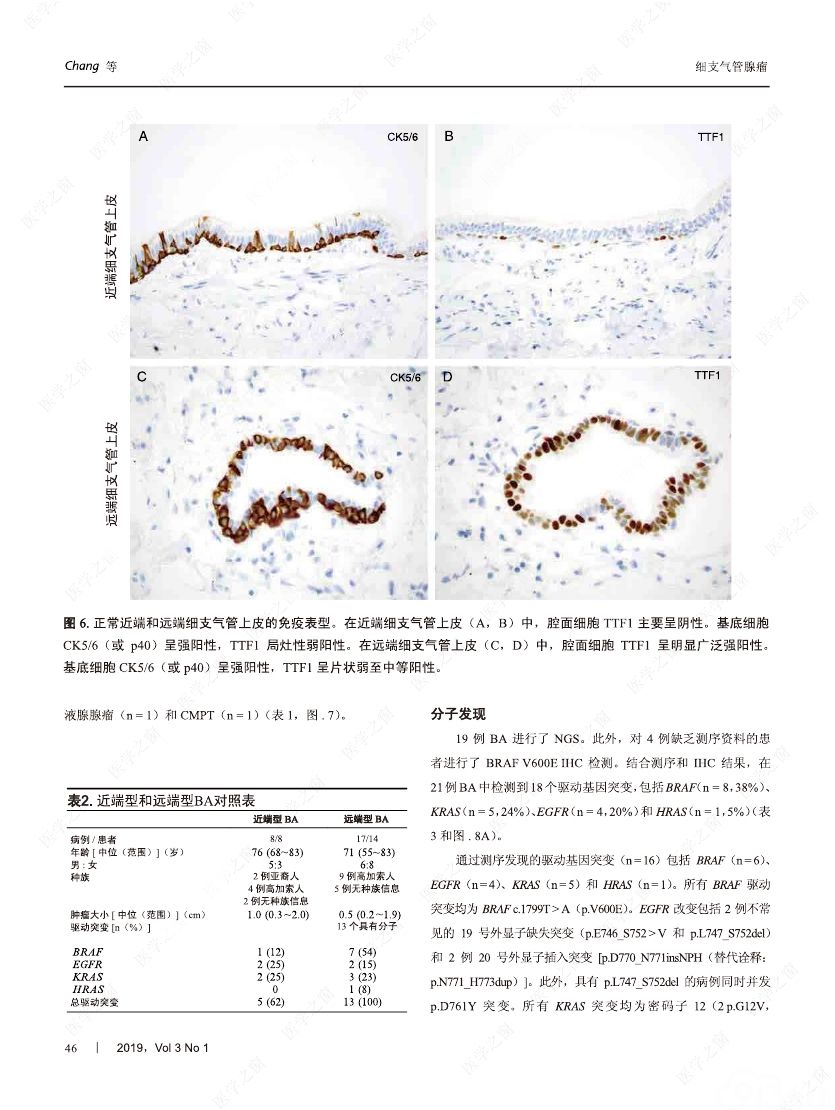
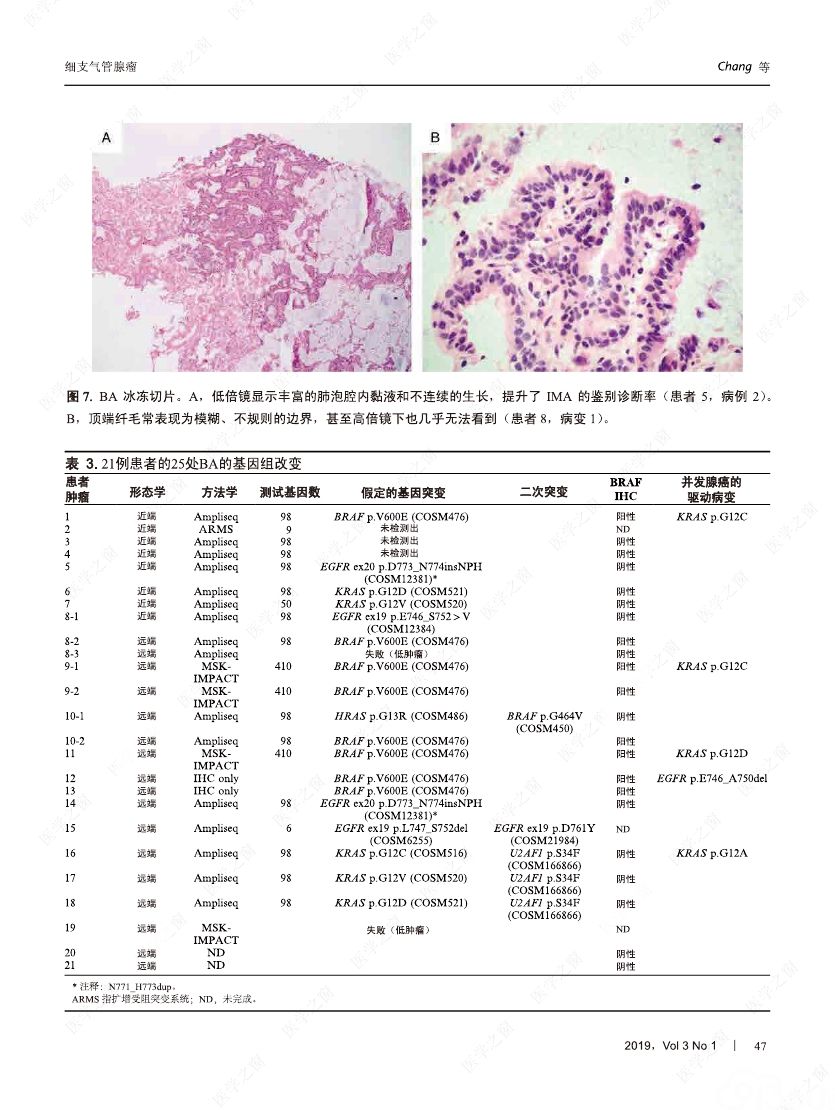
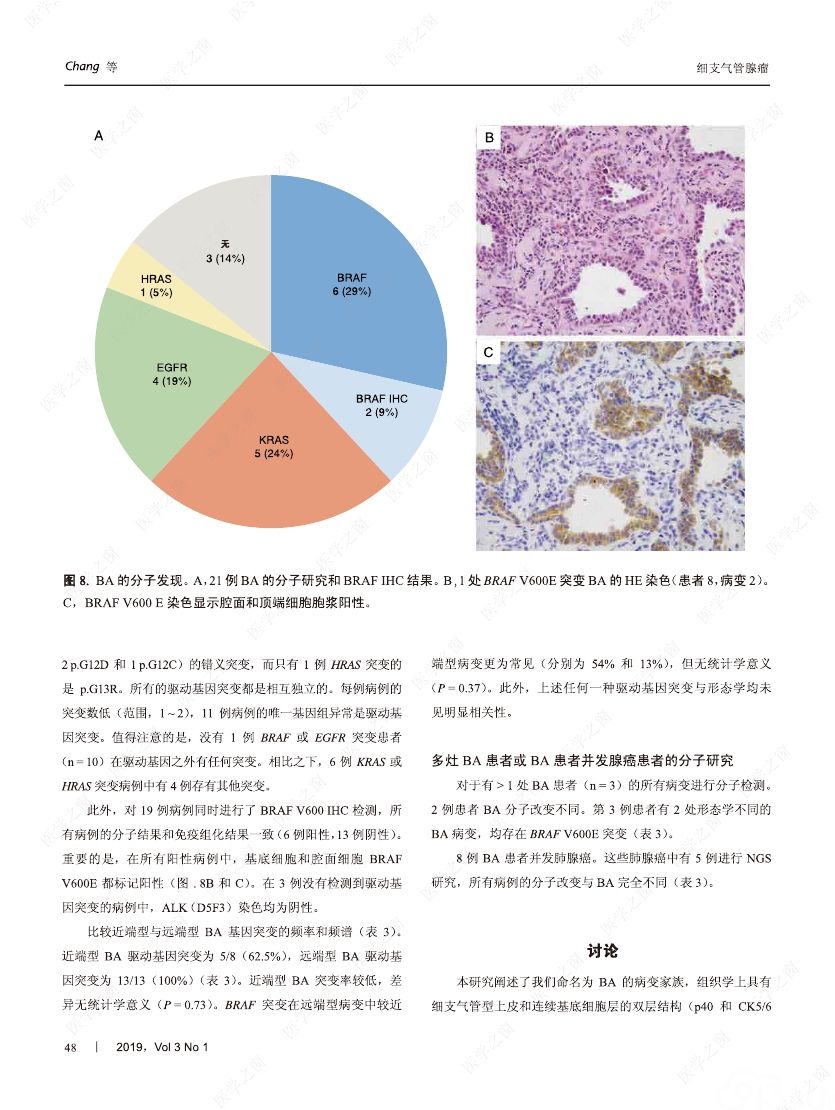
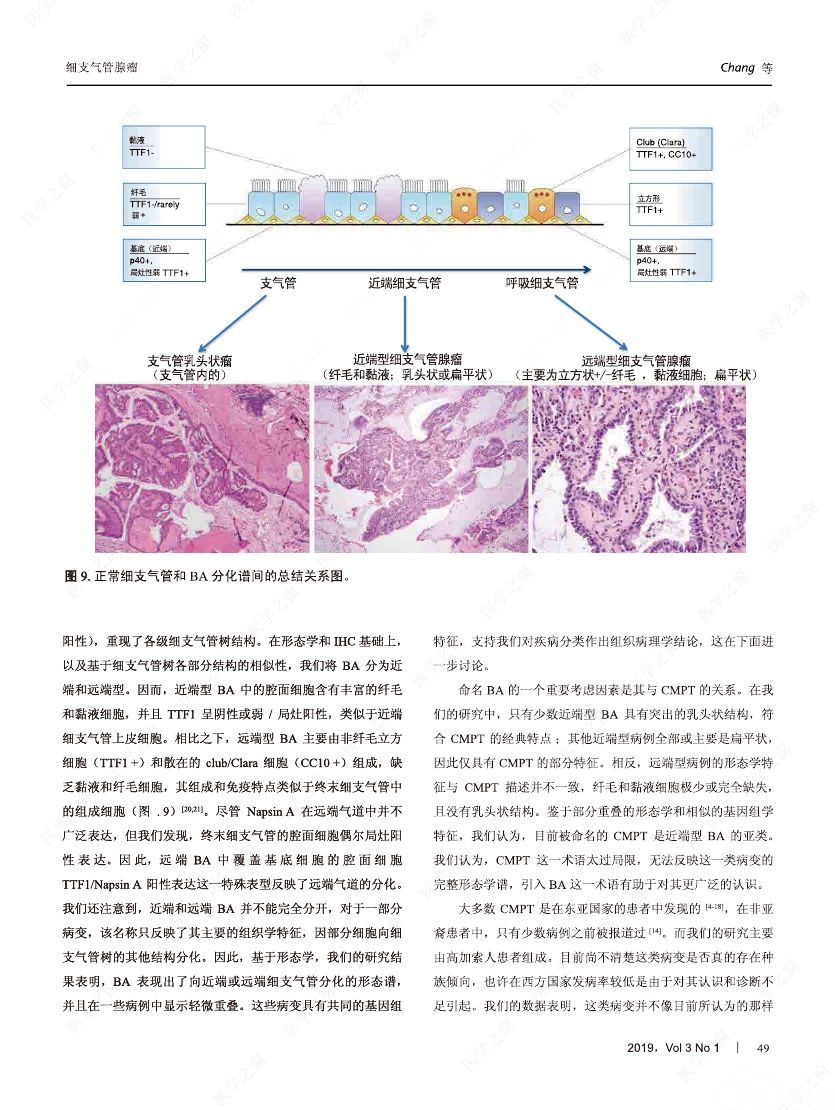
摘要:我们分析了25例累及肺实质的病变,具有以下病理特征:平滑的双层支气管型上皮发生结节状增生变化,包含连续的基底细胞层。这些病变与最近报道的肺纤毛黏液结节性乳头状肿瘤(ciliated muconodular papillary tumor,CMPT)具有一些相似的病理组织学特征;但大多数不完全符合CMPT诊断标准,因为仅有很少或无乳头状结构,且具有不同数量的纤毛和黏液性细胞,有的甚至完全缺失上述结构中的一种或全部。形态和免疫学表型呈现从类似近端细支气管(近端型:中度到丰富的黏液和纤毛细胞:管腔细胞中的TTF1阴性或弱阳性;n=8)到远端细支气管(远端型:少量或无黏液及纤毛细胞;管腔细胞中TTF1阳性;n=17)的特征。所有病变均有连续的基底细胞层(p40和CK5/6阳性)。我们将这些病变命名为细支气管腺瘤(bronchiolar adenoma,BA),并分析其临床病理和分子病理特征。所有的BA都是非连续的、境界清楚的病灶,病灶大小的中位直径为0.5cm(范围,0.2~2.0cm)。这些病变表现为完全平坦的有14处,含局灶乳头状结构的有7处,只有4处(均为近端型)有明显乳头状结构,符合CMPT的典型病理特点。值得注意的是,9处做过冰冻切片的病变中有7处被诊断为腺癌。术后未见任何病灶复发(中位随访,11个月)。对21例BA患者进行二代测序和/或免疫组化检测BRAF V600E,突变模式与之前描述的CMPT类似,包括:BRAF V600E突变(n=8,38%)、不常见的EGFR19号外显子缺失(n=2,10%)、EGFR20号外显子插入(n=2,10%)、KRAS突变(n=5,24%)和HRAS突变(n=1,5%)。近端型和远端型病变的突变模式相似。综上所述,我们描述了一类良性克隆增生的病变,其形态重现了各级分支的细支气管树结构,其中只有一小部分符合经典的CMPT。这些病变相似的突变模式和重叠的形态学特征支持同一疾病谱系。我们建议将这一类病变命名为BA,而CMPT是其一个亚型。
关键词:细支气管腺瘤,纤毛黏液结节性乳头状肿瘤,CMPT,双层,BRAF
AmJ Surg Pathol 2018;42:1010-1026
美国外科病理学杂志中文版2019年第1期全文No.5
欧春麟 翻译 周建华 审校







The American Journal of Surgical Pathology中文版声明:
©2018 Wolters Kluwer Health
The material is published by Wolters Kluwer Health with the permission of American Journal of Surgical Pathology.No part of this publication may be reproduced in any form,stored in a retrieval system or transmitted in any form,by any means,without prior written permission from Wolters Kluwer Health.Opinions expressed by the authors and advertisers are not necessarily those of the American Journal of Surgical Pathology, its affiliates,or of the Publisher.The American Journal of Surgical Pathology,its affiliates,and the Publisher disclaim any liability to any party for the accuracy,completeness,efficacy,or availability of the material contained in this publication (including drug dosages) or for any damages arising out of the use or non-use of any of the material contained in this publication.
Although advertising material is expected to conform to ethical (medical) standards,inclusion in this publication does not constitute a guarantee or endorsement of the quality or value of such product or of the claims made of it by its manufacturer.




 苏公网安备 32011402011742
苏公网安备 32011402011742